Treatment Updates
Emergency Use Authorization granted to 2 oral antivirals for COVID-19 treatment
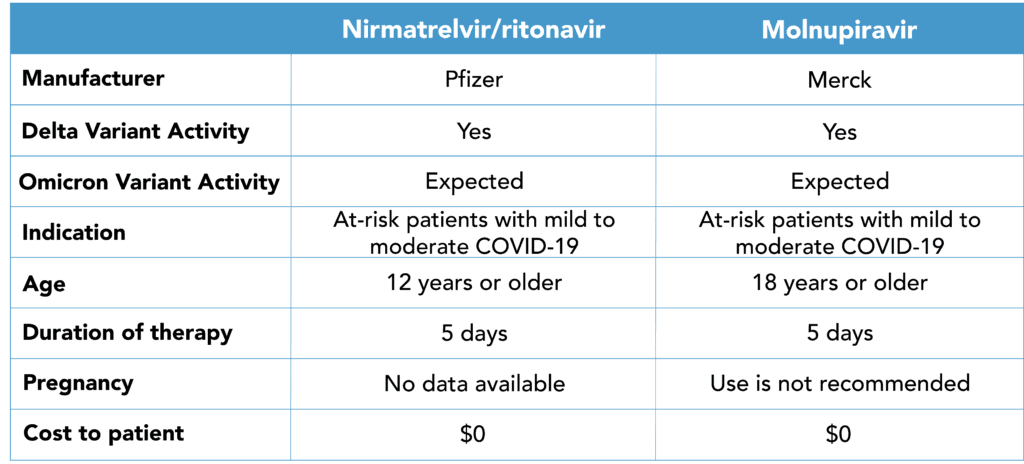
The FDA granted Emergency Use Authorization (EUA) to Pfizer’s Paxlovid (nirmatrelvir/ritonavir) and Merck/Ridgeback’s molnupiravir –the first two oral antivirals to treat COVID-19 at home. Prior to the FDA’s ruling, the only treatment available was injected or infused antibodies. Both of the newly authorized medications have shown to significantly reduce the risk of hospitalization or death when given within 3 days of symptom onset compared to placebo.
COVID-19 Oral Antiviral Reference Chart
When will these medications be available?
- As of January 3, 2022, the medications are shipping to pharmacies around the country. Both Pfizer and Merck are working diligently to increase the supply in order to satisfy the expected demand.
What is next on the treatment horizon?
- There are currently at least 10 medications in Phase III trials that are aimed towards treating COVID-19. Each of these drugs could prove useful treatments we have against COVID-19.