FDA Authorizes Novavax COVID-19 Vaccine, Adjuvanted
On July 13, 2022, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the Novavax COVID-19 Vaccine, Adjuvanted for the prevention of COVID-19 in individuals 18 years of age and older. This authorization offers adults who have not yet received a COVID-19 vaccination another option and adds another vaccine to the COVID-19 vaccine supply for the United States.
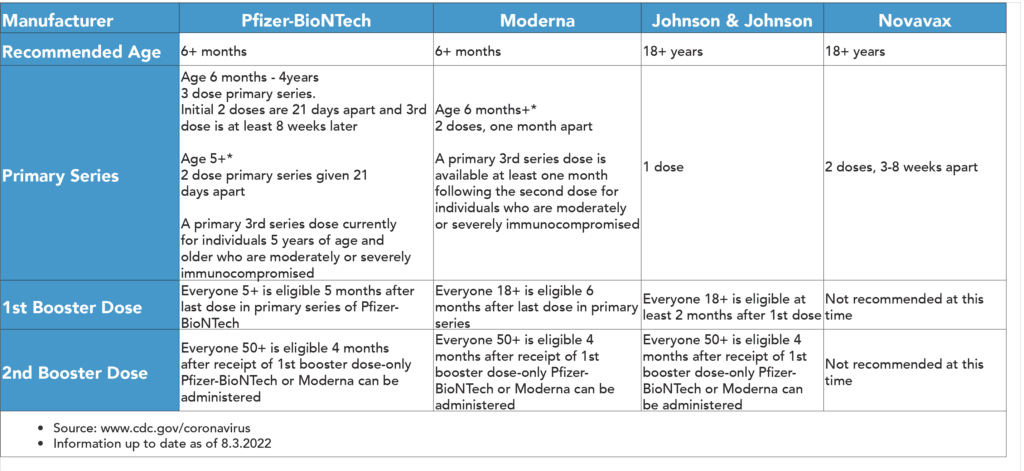
Click here for additional recommendations for immunocompromised people
View Previous Updates