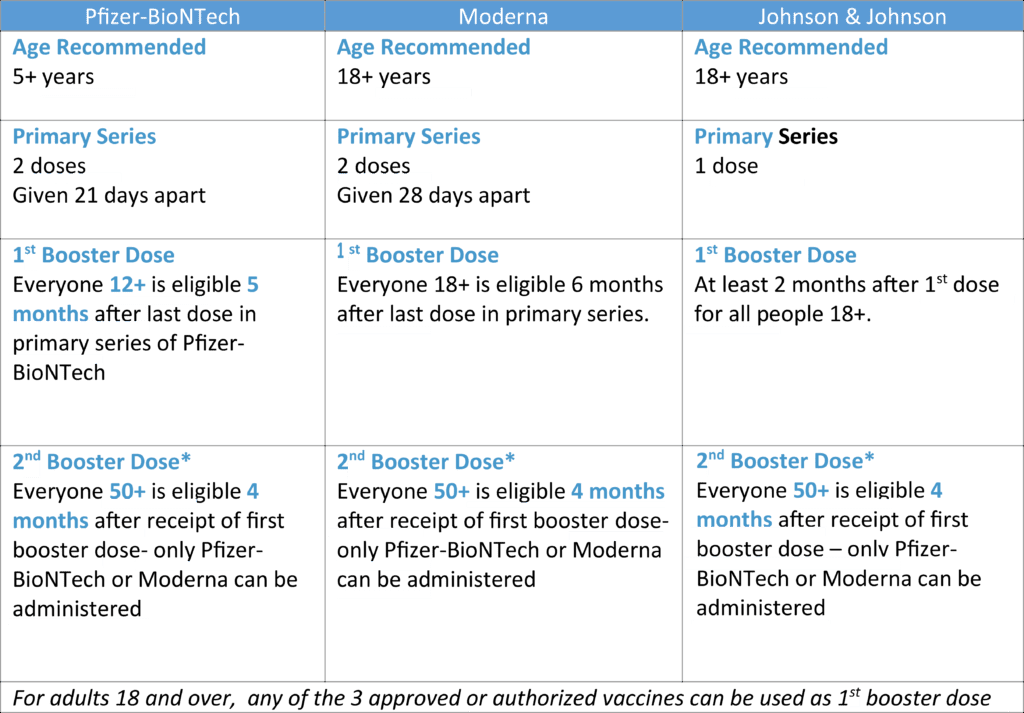
CDC Updates Recommendations to Allow for Second Covid-19 Booster for Certain Patient Groups
The FDA has amended the emergency use authorizations (EUAs) of Pfizer/BioNTech’s and Moderna’s COVID-19 vaccines to include a second booster dose for adults 50 years of age and older and certain immunocompromised individuals. The second booster doses should be administered at least 4 months after receipt of a first booster dose of any authorized or approved COVID-19 vaccine.
For certain immunocompromised individuals, the second booster dose of Pfizer/BioNTech’s vaccine is authorized for those 12 years of age and older, and the second booster dose of Moderna’s vaccine is authorized for those 18 years of age and older.
Click here for additional recommendations for immunocompromised people
View Previous Updates