FDA approves Pfizer-BioNTech COVID-19 Vaccine for children 5 thru 11
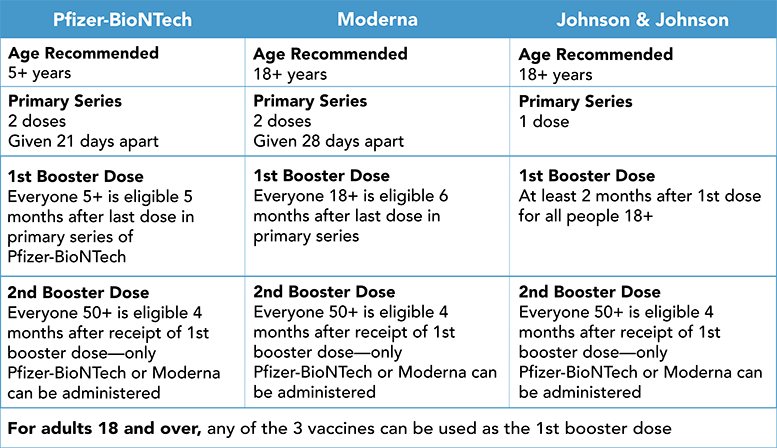
The FDA has amended the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine, authorizing the use of a single booster dose for administration to children ages 5 through 11 years of age at least 5 months after completion of a primary series of the Pfizer-BioNTech vaccine. This authorization is being put in place in order to provide continued protection against COVID-19 for the younger population.
Click here for additional recommendations for immunocompromised people
View Previous Updates